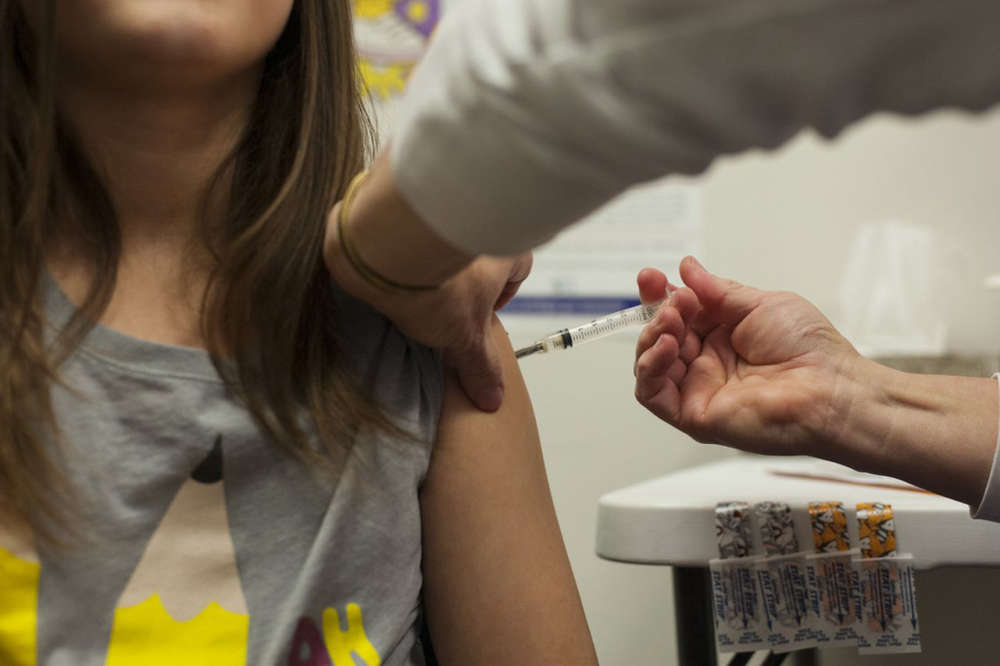
Pfizer’s COVID-19 vaccine for younger children could be authorized by the FDA this week and if approved, some could start receiving it within the next 2 weeks.
(WXYZ) — If the Pfizer’s COVID-19 vaccine gets approval this week, children between the ages of 5 and 11 could potentially start getting vaccinated in the first two weeks of November. In the meantime, the CDC says there’s one important thing to do while we wait.
Dr. Rochelle Walensky is talking about masks. She says, children are more vulnerable now to infection because of the more contagious Delta variant. Especially the kids who are attending in-person learning at school. She says having children wear masks is a very effective preventative measure to keep the virus from spreading.
Studies have shown that schools that don’t mask up are more likely to have COVID-9 outbreaks compared to schools that do require masks. Even if Pfizer‘s lower dose vaccine – which is 10 micrograms instead of 30 - is approved for kids 5 to 11, we should continue with preventative measures. As it’ll take some time for the vaccines to roll out and for kids to get the shots. She is excited that we’ll know soon if the vaccine is a go. The FDA’s independent vaccine advisory board is meeting to review Pfizer’s clinical data. If given the green light, then the CDC’s advisory board meets next week, Nov. 1 and 2 for their discussions.
Even if COVID case numbers continue to go down, we are heading into the winter months. This means more people are indoors and more opportunity for the virus to infect others.
Children in the U.S. make up about a quarter of the weekly reported COVID-19 cases.
Pfizer’s clinical trial data suggests that its vaccine was 90.7% effective against symptomatic disease in kids 5 to 11.

 8/5/24 - Flights Impacted Because Of Hurricane Debby
8/5/24 - Flights Impacted Because Of Hurricane Debby
 8/2/24 - U.S. / Russia Prisoner Exchange
8/2/24 - U.S. / Russia Prisoner Exchange
 7/31/24 - Lawmakers Consider Child Safety Bill
7/31/24 - Lawmakers Consider Child Safety Bill
 7/22/24 - President Biden Drops Out Of Presidential Race
7/22/24 - President Biden Drops Out Of Presidential Race


